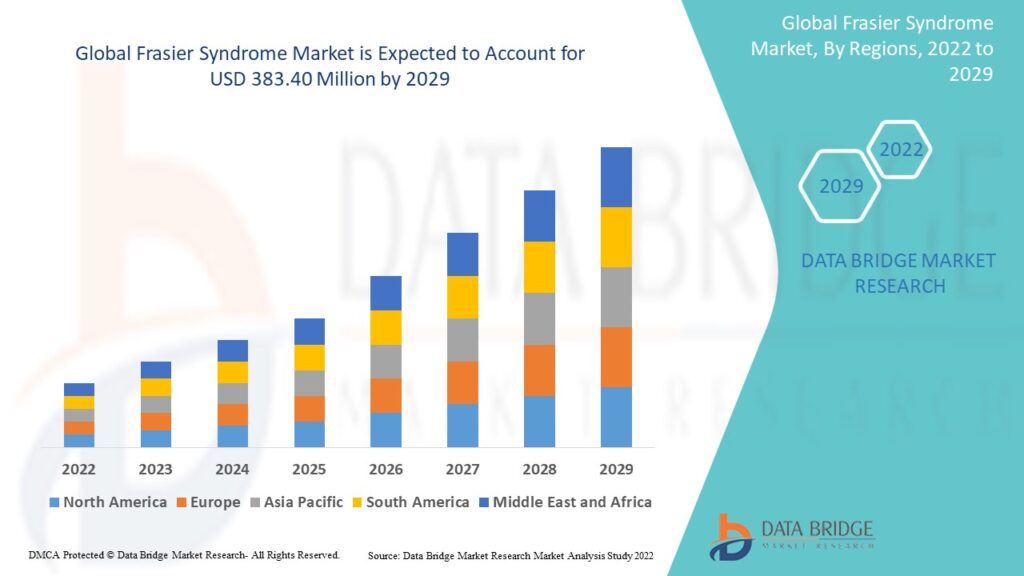
Introduction
The Frasier Syndrome Market is a specialized and evolving arena within the broader landscape of rare genetic diseases. Frasier syndrome, a condition first recognized in the late 20th century, stems from mutations in the Wilms tumor 1 (WT1) gene, a critical regulator of kidney and gonadal development. Individuals affected by this syndrome often experience progressive kidney dysfunction, gonadal dysgenesis, and an elevated risk of developing gonadoblastomas, a type of tumor associated with atypical gonadal tissue.
Despite its ultra-rare prevalence, the Frasier Syndrome Market has started to receive growing attention due to a confluence of factors: rising rare disease awareness, improvements in genetic testing technologies, increased patient advocacy, and a greater focus on personalized medicine. Though the number of affected patients remains exceedingly low, advancements in medical research, digital diagnostics, and international collaboration have widened the scope of both care and study. This market, while still embryonic in its commercial growth, embodies the future of precision healthcare—where even the rarest conditions are being addressed with sophistication, empathy, and cutting-edge science.
The Evolution
Early Discovery and Definition
Frasier syndrome was first differentiated from other nephrotic syndromes in the 1990s, thanks to advances in molecular genetics and a growing understanding of WT1 gene mutations. Initially mistaken for Denys-Drash Syndrome due to overlapping clinical presentations—including proteinuria, renal dysfunction, and gonadal abnormalities—Frasier syndrome gained recognition as its own entity with the identification of specific intronic mutations in WT1.
Shifts in Diagnostic Methodology
In its early years, diagnosing Frasier syndrome was a complex and slow process. Renal biopsy findings, hormonal imbalances, and ambiguous genitalia formed the triad of diagnostic clues. However, with the advent of next-generation sequencing (NGS), clinicians can now pinpoint WT1 mutations far earlier and more reliably.
Genetic counseling has since become a crucial component of the diagnosis journey, particularly for families with a history of unexplained kidney dysfunction. Prenatal diagnostics have also started playing a role in early identification, especially in high-risk cases with familial indications.
Multidisciplinary Management and Care
As understanding deepened, the approach to managing Frasier syndrome grew increasingly multidisciplinary. Pediatric nephrologists, endocrinologists, urologists, geneticists, and mental health professionals now form integrated care teams. This holistic management model is tailored not only to physical health but also to the psychological and social impacts experienced by patients, especially concerning gender identity, infertility, and the chronic nature of renal disease.
Market Trends
Several significant trends are reshaping the Frasier Syndrome Market, despite its limited patient base:
1. Precision and Genomic Medicine
The market is increasingly driven by genomics. Detailed genetic profiles allow for precision monitoring of disease progression and risk stratification. Some regions have begun integrating rare disease panels into newborn screening programs, facilitating earlier diagnosis and intervention.
2. Digital Health and Telemedicine
With specialists in rare genetic conditions often concentrated in urban centers or academic hospitals, telemedicine has emerged as a critical bridge. Remote genetic counseling, video consultations with nephrology experts, and virtual mental health support are reducing disparities in access.
3. AI and Predictive Modeling
Artificial Intelligence (AI) and machine learning are being applied to mine mutation databases, identify genotype-phenotype correlations, and predict patient outcomes. These tools are proving especially valuable in clinical decision support and research trial design.
4. Crowdsourced and Open-Access Research
Patient registries and open genomic platforms such as ClinVar and Orphanet allow researchers to share case data globally. This collaborative approach is vital for a disease with such low prevalence, helping to accelerate breakthroughs that would otherwise take decades.
5. Growth of Rare Disease Biotech
Niche biotech startups focusing solely on orphan diseases are entering the field. These companies often receive grants, tax incentives, and fast-track approvals from regulatory bodies like the FDA and EMA, encouraging innovation in therapeutic development.
Challenges
Despite promising trends, the Frasier Syndrome Market grapples with considerable challenges that hamper progress:
Ultra-Rare Patient Demographics
Frasier syndrome affects fewer than 1 in a million individuals, making patient recruitment for trials extraordinarily difficult. This demographic limitation also restricts revenue potential for pharma, making investment a hard sell without government or philanthropic incentives.
Misdiagnosis and Delayed Treatment
Symptoms such as nephrotic syndrome, delayed puberty, or ambiguous genitalia can lead to misdiagnosis. Some patients are initially treated for other kidney disorders, leading to suboptimal management and delayed genetic testing.
Absence of Targeted Therapies
There are currently no drugs specifically approved for treating Frasier syndrome. Treatment is symptomatic and includes renal replacement therapies like dialysis, hormone replacement therapy, and eventually kidney transplantation.
High Cost of Care
The cumulative costs associated with long-term renal care, hormone therapy, multiple surgeries, genetic testing, and psychological counseling are substantial. Many patients, particularly in low- and middle-income countries, do not have access to comprehensive insurance coverage.
Ethical and Social Complexities
Issues related to gender assignment, fertility preservation, and mental health need to be handled with extreme sensitivity. Ethical dilemmas—such as decisions around prophylactic gonadectomy due to tumor risk—pose challenges for families and clinicians alike.
Market Scope
Although still niche, the market scope for Frasier syndrome is quietly broadening across multiple dimensions:
- Geographical Reach: North America and Western Europe remain at the forefront in terms of diagnosis and research. However, emerging economies in Asia-Pacific, particularly India, South Korea, and Singapore, are rapidly integrating genetic testing into tertiary care, expanding the market’s geographical reach.
- Diagnostics Expansion: Hospitals and diagnostic labs are incorporating whole-exome and whole-genome sequencing, allowing for broader detection capabilities. National initiatives in the UK and Japan are pushing forward rare disease genomics frameworks at population scale.
- Care Infrastructure: Pediatric nephrology centers, genetic counseling hubs, and rare disease registries are increasing in number, enabling more comprehensive patient care and data-driven insights.
- Growth Trajectory: While specific forecasts are difficult to pin down due to the rarity, the broader rare disease segment—projected to grow by 4–6% CAGR globally—is a reasonable proxy for estimating Frasier Syndrome Market expansion, especially as part of genetic renal disorder portfolios.
Factors Driving Growth
There are several macro and micro-level forces contributing to the advancement of the Frasier Syndrome Market:
Global Rare Disease Policy Frameworks
Governments and health agencies are launching national plans to address rare diseases. These include funding for research, expansion of diagnostic capabilities, and orphan drug development incentives.
Advocacy and Patient Voice
Organizations like Global Genes, EURORDIS, and patient-led advocacy groups are vital in raising awareness, lobbying for funding, and creating support ecosystems for rare diseases like Frasier syndrome.
Academic and Clinical Research Funding
Universities and research hospitals are investing in long-term cohort studies. Grants from NIH, Horizon Europe, and philanthropic foundations are supporting novel investigations into WT1 mutation pathways and gene therapy candidates.
Biotechnology Innovations
Gene-editing technologies such as CRISPR/Cas9 are being explored for preclinical modeling of WT1 correction. These tools, though not yet applicable in humans for this condition, represent a futuristic pathway for eventual disease modification.
Global Registry Formation
Databases that track Frasier syndrome cases, genotype data, clinical outcomes, and interventions enable the scientific community to gain more clarity about disease trajectory and intervention efficacy.
Mental Health Integration
More comprehensive patient care models now integrate psychological support as a formal treatment pillar. Given the lifelong nature of the disease and its effect on gender identity, this represents a significant step toward holistic wellness.
Conclusion
The Frasier Syndrome Market reflects a new frontier in how modern medicine approaches ultra-rare conditions. Although the commercial incentives are limited compared to more common diseases, the stakes—measured in improved patient lives, early diagnosis, and reduced suffering—are enormous.
With the confluence of genetic research, global collaboration, digital health, and patient empowerment, there is a genuine momentum driving the evolution of this specialized market. While barriers such as high treatment costs, diagnostic delays, and the absence of curative options still loom large, the collective dedication of scientists, clinicians, patients, and policy-makers continues to chart a more hopeful path.
This market may be rare, but so is the determination of those involved. And with each advancement—whether scientific, clinical, or social—we move closer to ensuring that no condition, however rare, is left in the shadows.